Introduction: Patients with relapsed/refractory diffuse large B-cell lymphoma (r/r DLBCL) who are ineligible for intensive chemotherapy and autologous stem cell transplantation have limited therapeutic options and poor prognosis, and there is an unmet need for new therapeutic alternatives in this situation. Based on gene expression profiling, DLBCL can be divided into the germinal centre B-cell (GCB) and the activated B-cell (ABC) subtype. These subtypes are dependent on different oncogenic pathways and may differ in responsiveness to targeted therapies. Idelalisib is a small-molecule inhibitor of PI3Kδ, currently approved for treatment of follicular lymphoma and CLL. Based on the high response rate of idelalisib in heavily pretreated patients with indolent B-cell lymphomas, among whom many may have undetected transformed disease, we hypothesized that idelalisib may also be active in r/r DLBCL, particularly in the GCB subtype, due to frequent PTEN loss and unregulated activation of PI3K signaling in this subtype. Here, we evaluated the efficacy and safety of idelalisib as a single agent therapy in patients with r/r DLBCL in a multi-centre phase II non-randomized trial. To our knowledge, this is the first prospective study on the use of single agent idelalisib in patients with DLBCL.
Methods: Eligibility criteria were: Patients with DLBCL, including transformed low-grade lymphoma, with r/r disease after at least one rituximab-containing chemotherapy regimen, WHO PS 0-3, and not candidate for autologous stem cell transplantation. The primary endpoint was maximal overall response rate (ORR) measured with CT and PET/CT for the GCB-DLBCL. Idelalisib 150 mg x 2 p o was given until progression or unacceptable toxicity.
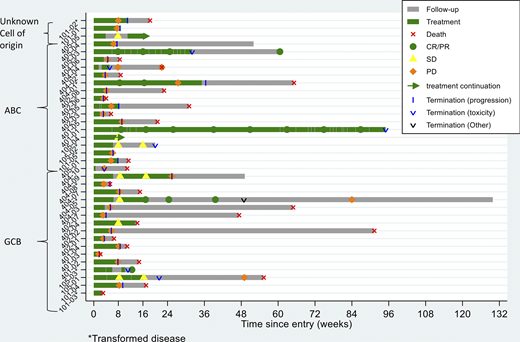
Results: In the period 2017-2020, 36 patients were included from six centers in Sweden and Denmark, 18 patients showed a GCB and 16 patients an ABC subtype, two patients could not be classified for subtype. The study was terminated prematurely due to futility in reaching the primary endpoint. The median age was 74 years. Patients had received a median of three previous regimens. In total, 34/36 patients have discontinued treatment (n=24 due to PD, n=7 due to an adverse advent (AE), n=2 due to death, n=1 due to other cause). Median duration of treatment was 8 weeks (range 2-92), see the swimmers plot. Treatment emergent-AEs of grade 3 or higher included elevated liver transaminases (n=6), hematological toxicity (n=4), colitis (n=2), CMV reactivation (n=1), and skin toxicity (n=1). No patient died due to possible treatment-related toxicity. With a median follow up time of 4.9 months, 22 patients were evaluable for efficacy after 8 weeks of treatment, as of May 31, 2020. Fourteen patients had progressive disease before that point of time. The ORR in all patients was 14 % with 3 patients achieving CR (8 %) and 2 patients PR (6 %). In GCB versus ABC subgroups, 2 patients (11 %) compared to 3 patients (19 %) patients reached CR/PR. Among the 9 patients with transformed lymphoma, 3 patients (33 %) reached CR/PR. Among the 5 patients with CR/PR, median duration of response was 67 weeks (95 % CI:19-not reached). Median OS in the GCB subgroup was 15 weeks (95 % CI: 11-56) compared to 23 weeks (95 % CI: 9-not reached) in the ABC subgroup. To date, 2 of the responding patients show ongoing response after 53 respectively 86 weeks of treatment.
Conclusions: Single agent idelalisib demonstrated modest activity in patients with r/r DLBCL, but the primary endpoint with higher activity in the GCB subtype was not reached. Instead, a numerically higher response rate was observed in the ABC subgroup. In addition, we noticed a higher number of responding patients among patients with transformed lymphoma compared to patients with primary DLBCL. The results need to be interpreted with caution given the low number of patients. Idelalisib showed a manageable and expected safety profile. Considering the limited treatment options for patients with r/r DLBCL, there is an urgent need for novel therapeutic approaches, especially for patients who are ineligible for autologous stem cell transplantation and chimeric antigen receptor T-cells (CART) therapy. Further exploration of the potential benefit of PI3K inhibitors in selected subgroups of r/r DLBCL is motivated. Translational analyses to reveal potential biomarkers for efficacy of PI3K inhibition in DLBCL will now be performed, in tumor tissue and in circulating tumor DNA from plasma.
Ekstroem Smedby:Celgene: Other: Advisory Board; Janssen Cilag: Research Funding; Takeda: Research Funding. Larsen:Roche: Other: Advisory Board; Gilead: Other: Advisory Board; Celgene/BMS: Other: Advisory Board; Novartis: Other: Travel grants, Advisory Board. Jørgensen:Gilead: Other: Advisory Board; Novartis: Other: Advisory Board; Roche: Other: Advisory Board. Brown:Gilead: Other: Advisory Board. Jerkeman:Celgene: Research Funding; Abbvie: Research Funding; Janssen: Research Funding; Gilead: Research Funding; Roche: Research Funding.
Idelalisib is a small-molecule inhibitor of PI3KÃŽÃ'´ currently used for patients with CLL and follicular lymphoma. In this trial, based on the high response rate in heavily pretreated patients with indolent B-cell lymphomas, we hypothesize that this agent may also be active in relapsed/refractory DLBCL.
Author notes
Asterisk with author names denotes non-ASH members.